
With the legacy media gleefully reporting on the FDA's full approval of Pfizer-BioNTech's COVID-19 vaccine now known as Comirnaty like this:
…and this:
…you might believe that the vaccine is now perfectly safe because it has been fully tested. What is missing from most legacy media reports can be easily found in the FDA's Biologics Licence Approval (BLA) dated August 23, 2021.
Let's start with the lead page of the FDA approval letter to BioNTech:
From this, it would appear that Pfizer has the full and unencumbered authorization necessary to manufacture and distribute Comirnaty.
Actually, on pages 4 and 5 and you will find this:
Despite the fact that govermemts around the world are vaccinating children as young as 12 years of age (and, in some cases, allowing children down to the age of 12 to make the decision on their own without parental input) should be concerning since even the FDA admits that studies for those under the age of 16 have not been completed no matter what your local public health official may tell you. As well, it is also important to note the following study completion and final report submission dates for each of the three age groups:
12 to 15 years – May 31, 2023 – final report October 31, 2023
6 months to 11 years – November 30, 2023 – final report May 31, 2024
Ages less than 6 months – July 31, 2024 – final report October 31, 2024
In addition to these studies, Pfizer is obligated to complete additional studies for other health issues including myocarditis and pericarditis as shown on page 6, 7 and 8:
Please note the following study completion dates keeping in mind that the submission of final reports are not required for several months after the study completion dates:
1.) The study on any links between the Pfizer-BioNTech COVID-19 mRNA vaccine and myocarditis and pericarditis will not be complete until June 30, 2025.
2.) The study on any links between Comirnaty and myocarditis and pericarditis will not be complete until March 31, 2024.
3.) The substudy describing the natural history of myocarditis and pericarditis following administration of Comirnaty will not be complete until March 31, 2024.
4.) The prospective cohort study with at least five years of follow-up for potential long-term sequelae (a condition which is the consequence of a previous disease or injury) will not be complete until December 31, 2026.
5.) The substudy which assesses the incidence of subclinical myocarditis following the administration of a second dose of Comirnaty in participants aged 5 to 15 years will not be complete until November 30, 2023.
6.) The substudy which assesses the incidence of subclinical myocarditis following the administration of a third dose of Comirnaty in participants aged 16 to 30 years will not be complete until June 30, 2022.
As you can see, with nearly 170 million Americans already fully vaccinated these studies, which would normally have been done before the vaccine was unleashed on the public, will not see the light of day for between one and five years from now by which time the potential health damage will already have been done. It will be a prime example of closing the barn door after the horse escapes.
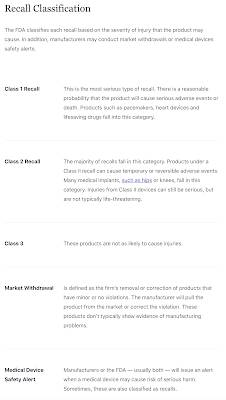
Remember, FDA approval does not mean that a drug is safe. The FDA has a long track record of approving pharmaceutical products that have proven to be hazardous to human health and even has a classification for the recalls that are based on the severity of injury that is caused by the drug or device as shown here:
According to DrugWatch, on average, 4,500 drugs and devices are recalled by the FDA every year even though the products have received the FDA "seal of approval". Here is a list of just a few of the drugs that were recalled with many having been used for decades before they were pulled from the market:
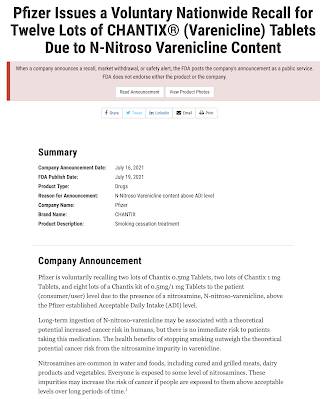
Just for fun, here is a very recent recall of a Pfizer product:
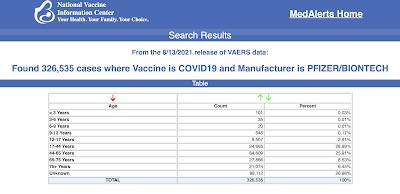
God forbid that the legacy media actually take a few minutes and read through the FDA BLA approval document and actually report on the details contained in its eleven pages, many of which are very important given the rapid rollout of the COVID-19 vaccines, the hundreds of thousands of reports of adverse reactions to the Pfizer-BioNTech vaccine in the United States alone as shown here:
…and the fact that many nations are already planning to roll out a third dose of Big Pharma's latest venture as well as making it mandatory for certain groups of people to be jabbed.
Let's close this posting with this article that appeared on the British Medical Journal website on August 20, 2021:
Here are key quotes from the article with bolds being mine:
"Transparency advocates have criticised the US Food and Drug Administration’s (FDA) decision not to hold a formal advisory committee meeting to discuss Pfizer’s application for full approval of its covid-19 vaccine.
Last year the FDA said it was “committed to use an advisory committee composed of independent experts to ensure deliberations about authorisation or licensure are transparent for the public.” But in a statement, the FDA told The BMJ that it did not believe a meeting was necessary ahead of the expected granting of full approval.
“The FDA has held numerous meetings of its Vaccines and Related Biological Products Advisory Committee (VRBPAC) related to covid-19 vaccines, including a 22 October 20202 meeting to discuss, in general, the development, authorisation, and licensure of covid-19 vaccines,” an FDA spokesperson said.
“The FDA also has held meetings of the VRBPAC on all three covid-19 vaccines authorised for emergency use and does not believe a meeting is needed related to this biologics license application.”
The spokesperson added, “The Pfizer BioNTech covid-19 vaccine was discussed at the VRBPAC meeting on 10 December 2020.3 If the agency had any questions or concerns that required input from the advisory committee members we would have scheduled a meeting to discuss.”…
Kim Witczak, a drug safety advocate who serves as a consumer representative on the FDA’s Psychopharmacologic Drugs Advisory Committee,4 said the decision removed an important mechanism for scrutinising the data.
“These public meetings are imperative in building trust and confidence especially when the vaccines came to market at lightning speed under emergency use authorisation,” she said. “The public deserves a transparent process, especially as the call for boosters and mandates are rapidly increasing. These meetings offer a platform where questions can be raised, problems tackled, and data scrutinised in advance of an approval.”
Witczak is one of the more than 30 signatories of a citizen petition5 calling on the FDA to refrain from fully approving any covid-19 vaccine this year to gather more data. She warned that without a meeting “we have no idea what the data looks like.”
“It is already concerning that full approval is being based on 6 months’ worth of data despite the clinical trials designed for two years,” she said. “There is no control group after Pfizer offered the product to placebo participants before the trials were completed.
“Full approval of covid-19 vaccines must be done in an open public forum for all to see. It could set a precedent of lowered standards for future vaccine approvals.”"
You can publish this article on your website as long as you provide a link back to this page.









Be the first to comment