This article was last updated on April 16, 2022
Canada: ![]() Oye! Times readers Get FREE $30 to spend on Amazon, Walmart…
Oye! Times readers Get FREE $30 to spend on Amazon, Walmart…
USA: ![]() Oye! Times readers Get FREE $30 to spend on Amazon, Walmart…
Oye! Times readers Get FREE $30 to spend on Amazon, Walmart…
On May 4, 2021, Pfizer held its earnings call for the fiscal period ending March 31, 2021. With the world's leaders jumping through all kinds of hoops to get access to Pfizer's BNT162b COVID-19 vaccine, some of the details of the earnings call should be of interest to all of us given that we are participating in an unprecedented experiment for Big Pharma with a vaccine that has been incompletely tested on humans.
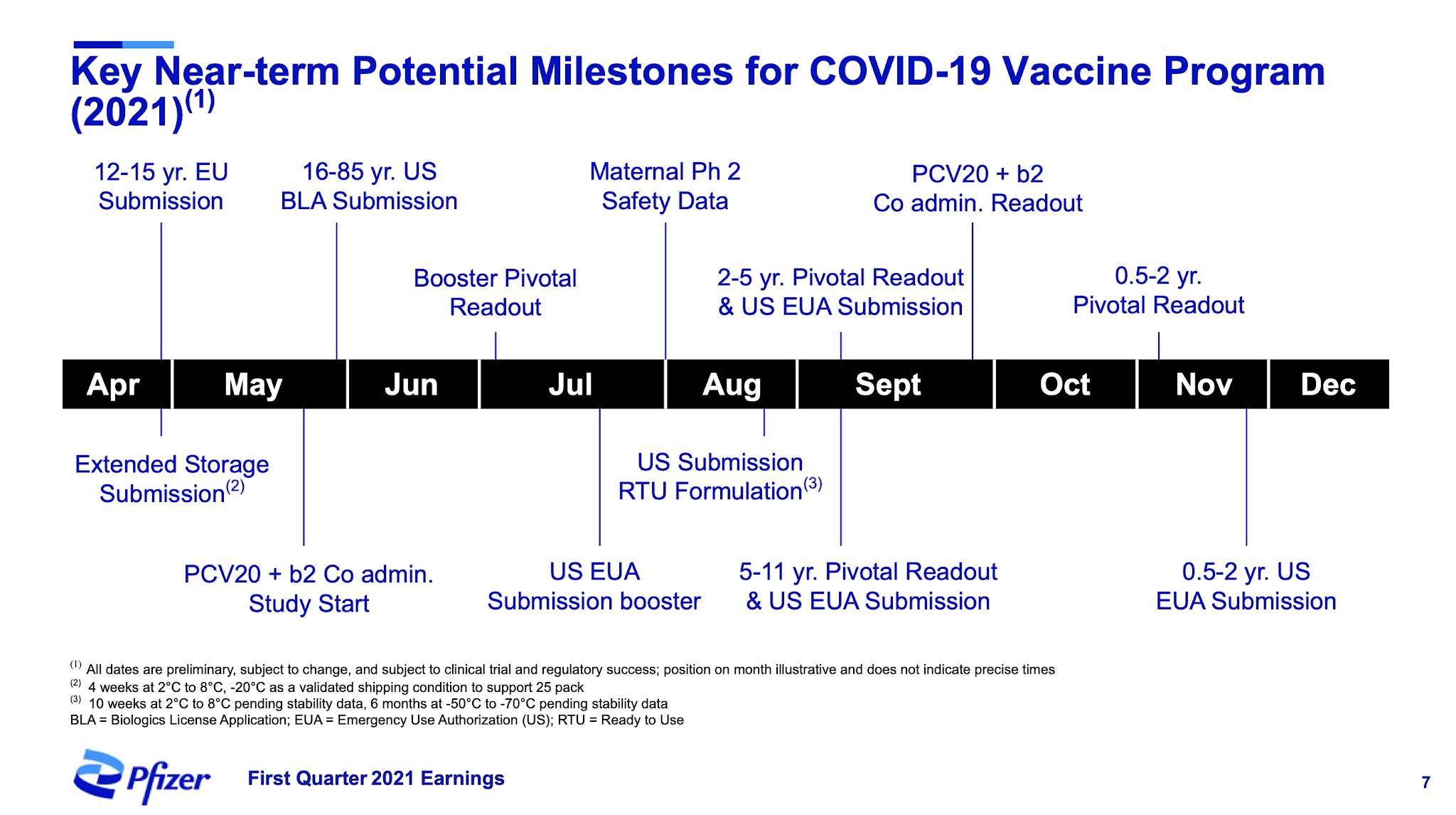
Let's start with this graphic from the presentation which shows Pfizer's first quarter revenue highlights:
As you can see, the Pfizer-BioNTech mRNA COVID-19 vaccine is responsible for almost as much revenue as the company's next four best-performing pharmaceuticals, hitting $3.462 billion compared to $3.879 billion for Eliquis, Ibrance, Xeljanz and Ibrance combined.
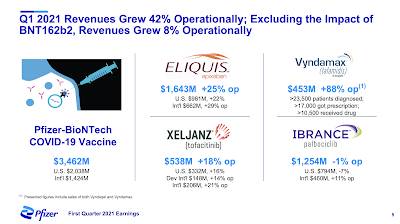
Here is a graphic showing the quarterly income statement highlights, noting that most metrics have been impacted by the BNT162b2 vaccine:
In its update on the BNT162b2 vaccine, the company expects that revenues will total roughly $26 billion in 2021 up from an earlier estimate of $15 billion, reflecting the 1.6 billion doses that will be delivered over the year under contracts signed to mid-April 2021. The company expects to have the capacity to manufacture at least 3 billion gnoses of the vaccine in 2022 compared to 2.5 billion in 2021. Just in case you thought that the pandemic would wind down over the next two or three months, Pfizer also claims that it is negotiating contracts with multiple governments for potential BNT162b2 vaccines for 2022 and beyond with recently signed contracts as follows:
– United Kingdom – 60 million additional doses in 2021
– Israel – unspecified millions of doses in 2022
– Canada – up to 125 million doses over the period from 2022 and 2023 and an additional option to supply up to 60 million doses in 2024.
To put the $26 billion in revenues from BNT162b2 into context, Pfizer expects total year 2021 revenues totalling between $44.6 billion to $46.6 billion for its product lines excluding BNT162b2. Operational revenues grew by 42 percent including its entire product line; when BNT162b2 is excluded, operational revenue grew by only 8 percent.
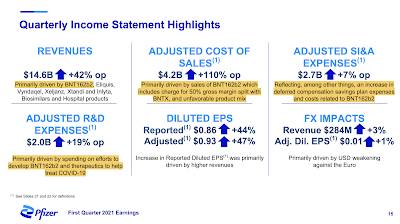
Here is a graphic from the presentation showing the near-term milestones for the COVD-19 vaccine:
This is clear proof that the vaccine is still in its experimental stage particularly given that Pfizer is applying for continued Emergency Use Authorizations (EUAs) from the United States government despite what our local public health officials may be telling us. Pfizer is also touting the fact that it has requested amendments to the United States EUA of BNT162b2 which would allow it to expand its unofficial trial to adolescents between the ages of 12 and 16 years, now has in place a paediatric study involving children from six months of age and a study involving pregnant women as shown in this quote from Pfizer's Chairman and CEO, Albert Bourla:
"We expect to hear back shortly from the FDA on our application for expanded Emergency Use Authorization for our COVID-19 vaccine to include individuals 12 to 15 years of age.
The Pfizer-BioNTech pediatric study evaluating the safety and efficacy of our COVID-19 vaccine in children six months to 11 years of age is ongoing. We expect to have definite readouts and submit for an EUA for two cohorts, including children two to five years of age and five to 11 years of age in September. The readout and submission for the cohort of children six months to two years old are expected in the fourth quarter. We also expect to have Phase 2 safety data from our ongoing study in pregnant women by late July, early August."
Pfizer is also evaluating the safety and immuongenecity of a third dose of the existing formulation of the COVID-19 vaccine for SARS-CoV-2 variants as quoted here:
"We are evaluating the safety and immunogenicity of a third dose of the existing formulation of our COVID-19 vaccine to understand the effect of a booster on immunity against the SARS-CoV-2 variants in circulation. Additionally, we have started an evaluation of an updated, prototype variant version of our vaccine that encodes the spike protein of the lineage B.1.351 SARS-CoV-2 variant, which includes the mutation E484K, first identified in South Africa.
This study is designed to establish a regulatory pathway to update the current vaccine to address any future variant of potential concern in approximately 100 days, if needed. We expect to have immunogenicity data for both studies in early July."
Pfizer's experiment with unprecedented vaccines is not stopping with the BNT162b2 vaccine. As shown on this slide, Pfizer is planning to test its mRNA vaccines for the upcoming seasonal influenza season even though the medium- and long-term impacts of injecting humans with RNA are not completely understood:
Here is a quote from Albert Bourla about the company's new product line:
"Pfizer has emerged as a leader in mRNA development, and we are exploring a wide range of opportunities for the technology. We are making rapid progress with our potential flu mRNA program, and we aim to maintain mRNA leadership with two potential game-changing mRNA approaches to a flu vaccine expected to enter the clinic in the third quarter of 2021. We will test multiple constructs in Phase 1, 2 to facilitate swift selection of an optimal tetravalent flu product dose regimen. We aim to develop initially a tetravalent flu vaccine using the modified mRNA platform.
Pending the generation of favorable immune and tolerability Phase 1, 2 data, a potential rapid progression to Phase 3 is possible given our large-scale pharmaceutical science and manufacturing capabilities. We are also exploring the potential to address other infectious diseases that we plan to discuss in the near future. In addition to prophylactic vaccines for infectious diseases, we believe mRNA has the potential to address a wide range of therapeutic areas, including cancer and genetic disease. As you have seen, today, we have increased our 2021 R&D guidance to reflect our plans to increase our mRNA capabilities, build momentum in our targeted areas of interest and deliver on mRNA's breakthrough potential for the benefit of people worldwide."
I like that, "for the benefit of people worldwide". Just in case you were curious, Pfizer paid out $2.2 billion in cash dividends tdo shareholders during the first quarter of 2021 so at least someone other than Pfizer's corner office dwellers is getting rich from the pandemic.
Let's close with this thought – at least the COVID-19 pandemic has been of significant financial benefit to one sector of the economy even as mom and pop businesses have suffered and permanently closed under government-mandated orders.
You can publish this article on your website as long as you provide a link back to this page.


Be the first to comment