
A recent interview with Pfizer CEO Albert Bourla on CNBC is quite eye-opening particularly given the very rapid growth in the number of COVID-19 cases around the world, particularly among the vaccinated.
Here is the interview with Meg Tirrell:
Here is a partial transcript of the interview with my bolds:
MT – "What is your expectation in terms of whether we're going to see an update to that vaccine that we just spoke of with Stephane Buncell from Moderna in the last hour who suggested, really the focus is on the fall for figuring out the right strains for them but, of course, we're already seeing Israel giving fourth booster doses so what do you think the future holds in terms of when we'll be getting the next boosters and what those boosters are going to contain?"
AB – "I wouldn't say that the future is clearly predictable right now but what it think it is that we are doing everything we can so that we can stay ahead of the virus and let me start with I don't know if there is a need for a fourth booster, that's something that needs to be tested and I know that Israel already started some of these experiments and we will conduct also some of these experiments to make sure that if needed we use it. I don't think we should do anything that is not needed. Also, we are working on a new version of our vaccine, a version that will be effective against Omicron as well that will not be effective against the other variants but against Omicron as well. The hope is that we will achieve something that will have way, way better particularly against infections because the protection against the hospitalizations and the severe diseases it is….reasonable right now with the current vaccines as you are having, let's say, the third dose. This vaccine will be ready in March. I don't know if we will need it. I don't know if and how it will be used but will be ready, in fact, we already starting manufacturing some of these quantities of threes so if there is a need for that vaccine that we will have some immediately because there are a lot of governors that would like to see it immediately. And clearly…this is where most of the efforts of most of the governments is moving."
I found it interesting that he claimed that Pfizer's COVID-19 vaccine provides "reasonable protection" once you have the third dose. This is coming from a company that claimed that their product was 95 percent effective when it was first approved. And, just as we all suspected, Israel is ground zero for Pfizer's experiment with its COVID-19 vaccine. His comments on the timing of Pfizer's new Omicron-specific " concoction" are also interesting given that governments around the world are heavily promoting a booster with the same old vaccine formulation that has, by Bourla's own admission, provided only "reasonable" protection.
Here's a graphic from the United Kingdom's Health Security Agency dated December 31, 2021 showing the vaccine effectiveness against both the Delta and Omicron variants plotted against time in weeks since being fully vaccinated with three doses of Pfizer's BNT162b2 and Moderna's mRNA-1273:
I would think that a vaccine effectiveness of only 60 percent for 2 doses of Pfizer's vaccine for the Delta variant at 25 weeks or more and a vaccine effectiveness of only 50 percent against Omicron at 10 weeks or more can hardly be termed "reasonable". But, then again, my expectations might be too high.
After a discussion about the company's new Paxlovid antiviral product, he also weighs in on the so-called "antivaxxers" when asked whether breakthrough cases of Omicron are going to have an impact over the longer term for boosters.
"I think this is a real risk because, you know, there is always the element of people who could get tired but I believe that this situation has unfortunately not fortunate but unfortunately a form to camps to different mindsets. There is a mindset that they are very fanatic that they are don't want vaccine and there's a mindset of other people they want maximum protections I believe in the element of in this segment of the people that they do believe in the value of the vaccine the people that they want maximum protection I think they will follow at large the instructions of the healthcare authorities and their physician. The other camp which is the ones that they are very skeptical I think they will remain skeptical and for the, I think unfortunately the solution only will be the pill if they get diseased. And then there is in between which is the the number of people which is smaller this segment that it can go one way or another and this is where education needs to happen."
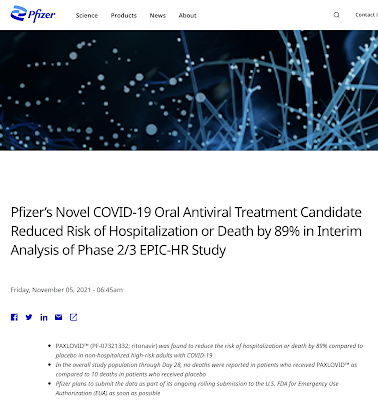
If its maximum protection that people are looking for they will be sadly disappointed in the degree of protection that the mRNA vaccines are providing. In addition, according to Bourla, the relatively small minority of people that are skeptical about Pfizer's rushed-to-market COVID-19 vaccine will have only Pfizer's rushed-to-market COVID-19 antiviral pill to protect them from certain death. Interesting, this drug is also touted to have a risk reduction for hospitalization and death of 89 percent, only slightly less than its "highly successful" BNT162b2 vaccine as shown here:
In case you were curious, the Phase 2/3 trial for PAXLOVID used 1219 adults who were enrolled by September 29, 2021 and yet, despite the very short trial, the FDA granted the company an Emergency Use Authorization for PAXLOVID on December 22, 2021.
You can publish this article on your website as long as you provide a link back to this page.


Be the first to comment