A brief article in the British Medical Journal (BMJ), one of the world's leading medical publications, by Associate Editor, Peter Doshi, puts the current mainstream media giddiness about the prospect of a COVID-19 vaccine into perspective, information that you will not hear from your news reader, public health official or politician.
As background, let's look at Peter Doshi's background to help us put his qualifications into perspective:
Doshi's article entitled "Will covid-19 vaccines save lives? Current trials won't tell us" provides us with background information on the main players in the COVID-19 vaccine "game" and the ultimate effectiveness of vaccines once phase III trials are complete. Obviously, to those of us that are laypersons, we expect that an approved vaccine will be safe and that it will prevent people from getting very ill and dying. As well, the layperson would expect that a vaccine will interrupt the cycle of infection, meaning that there will be no need for the seemingly endless cycles of lockdowns.
All of that said, Doshi's thesis is as follows:
"…the current phase III trials are not actually set up to prove either. None of the trials currently under way are designed to detect a reduction in any serious outcome such as hospital admissions, use of intensive care, or deaths. Nor are the vaccines being studied to determine whether they can interrupt transmission of the virus."
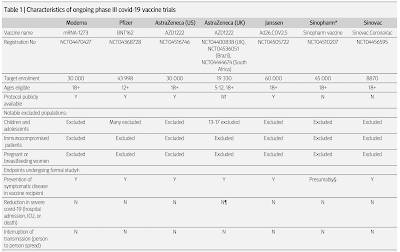
Doshi provides us with a table showing the characteristics of ongoing phase III COVID-19 vaccine trials (effective to October 2020) noting that certain populations are excluded from most of the trials as follows:
1.) Children and adolescents
2.) Patients with compromised immune systems
3.) Pregnant and breastfeeding women
As well, all of the current studies are only designed to study the prevention of symptomatic disease in those who receive the vaccine, they are not studying the following:
1.) a reduction in severe cases that would result in admission to a hospital, ICU or death
2.) interruption in person-to-person spread (i.e. transmissibility)
Here is the table:
Let’s close this posting with another quote from a longer version of the same article written by Peter Doshi:
“In a September interview Medscape editor in chief Eric Topol pondered what counts as a recorded “event” in the vaccine trials. “We’re not talking about just a PCR [polymerase chain reaction test]-positive mild infection. It has to be moderate to severe illness to qualify as an event, correct?” he asked.
“That’s right,” concurred his guest, Paul Offit, a vaccinologist who sits on the FDA advisory committee that may ultimately recommend the vaccines for licence or emergency use authorisation.
But that’s not right. In all the ongoing phase III trials for which details have been released, laboratory confirmed infections even with only mild symptoms qualify as meeting the primary endpoint definition. In Pfizer and Moderna’s trials, for example, people with only a cough and positive laboratory test would bring those trials one event closer to their completion. (If AstraZeneca’s ongoing UK trial is designed similarly to its “paused” US trial for which the company has released details, a cough and fever with positive PCR test would suffice.)
Part of the reason may be numbers. Severe illness requiring hospital admission, which happens in only a small fraction of symptomatic covid-19 cases, would be unlikely to occur in significant numbers in trials. Data published by the US Centers for Disease Control and Prevention in late April reported a symptomatic case hospitalisation ratio of 3.4% overall, varying from 1.7% in 0-49 year olds and 4.5% in 50-64 year olds to 7.4% in those 65 and over.13 Because most people with symptomatic covid-19 experience only mild symptoms,14 even trials involving 30 000 or more patients would turn up relatively few cases of severe disease.
In the trials, final efficacy analyses are planned after just 150 to 160 “events,”—that is, a positive indication of symptomatic covid-19, regardless of severity of the illness.” (my bolds)
This may go a long way to explaining why Big Pharma is releasing test results that show vaccine effectiveness ranging from 90 to 95 percent, effectiveness that may not be achievable in real world conditions.
The next time that you hear a politician, public health official or one of the bobbing heads in the mainstream media shilling for Big Pharma and its "miracle COVID-19 vaccine", please keep this information in mind. We may not be getting what we are hoping for in a vaccine and, as history shows, there can well be serious adverse reactions from vaccines that are brought to market during periods of pressure for Big Pharma to deliver as shown here:
Remember this. In nearly the words of 1930s and 1940s Germany, "Vakzin macht Frei". Go ahead, look it up.
Click HERE to read more from this author.
You can publish this article on your website as long as you provide a link back to this page.



Be the first to comment