With the novel strain of the coronavirus making the news cycle on a daily basis, the search for a new universal influenza vaccine is particularly pertinent.
When we think about a global pandemic, the first thing that comes to mind is the Spanish H1N1 influenza pandemic of 1918 that led to the deaths of 50 million people worldwide and an estimated 675,000 Americans. The most unusual characteristic of this strain of the influenza virus was the high death rate that it caused among healthy 15 to 34 year olds, a factor that led to a 12 year decrease in the life expectancy in the United States. A comparable death rate from influenza has not taken place prior to or since the 1918 pandemic. Despite the improvements in medical care over the past century, there is no guarantee that such an influenza-related pandemic will not occur again, particularly given the ease of global mass travel in the 21st century.
As it stands now, if humans wish to have some protection against influenza viruses, an annual influenza vaccination is required. The annual vaccine only protects us from the influenza viruses that researchers predict will be the most common during the normal flu season. The vaccine causes antibodies to develop in the human body roughly 14 days from vaccination. Most of the vaccines provide protection for four viruses (quadrivalent); two influenza A viruses (H1N1 and H3N2) and two influenza B viruses. Other vaccines provide protection for three viruses (trivalent); two influenza A viruses (H1N1 and H3N2) and one influenza B virus. The trivalent vaccine is designed specifically for adults aged 65 and older since it creates a stronger immune response. The vaccine causes antibodies to develop in the human body roughly 14 days from vaccination The vaccine does not protect us from new forms of the influenza virus which is problematic given the rapidly mutating nature of the virus.
Here is a listing of the types of influenza vaccines currently available:
1.) Trivalent influenza vaccines:
A trivalent influenza shot made with adjuvant (Fluad), licensed for people 65 years and older.
A high-dose influenza vaccine (Fluzone High-Dose), licensed for people 65 years and older.
2.) Quadrivalent flu vaccines:
Standard-dose quadrivalent influenza shots that are manufactured using virus grown in eggs. These include Afluria Quadrivalent, Fluarix Quadrivalent, FluLaval Quadrivalent, and Fluzone Quadrivalent. Different influenza shots are licensed for different age groups. Some are licensed for children as young as 6 months of age. Most influenza shots are given in an arm muscle with a needle. One quadrivalent influenza shot (Afluria Quadrivalent) can be given either with a needle (for people aged 6 months and older) or with a jet injector (for people aged 18 through 64 years only).
A quadrivalent cell-based influenza shot (Flucelvax Quadrivalent) containing virus grown in cell culture, which is licensed for people 4 years and older. This season, all four of the vaccine viruses used in Flucelvax have been grown in cells, making the vaccine totally egg-free.
Recombinant quadrivalent influenza shot (Flublok Quadrivalent), an egg-free vaccine, approved for people 18 years and older.
If a universal influenza vaccine was invented, it would provide broader protection from a wider range of influenza viruses. This protection would also last more than a year, potentially requiring fewer inoculations. Let's look at two examples of groups that are developing this critically-needed protection.
Acccording to this report from the National Institute of Allergy and Infectious Diseases (NIAID), the first clinical trial of a universal universal influenza vaccine on human subjects is taking place. The experimental vaccine was develop by NIAID and is known as H1ssF-3928:
The Phase 1 clinical trial is taking place at the National Institute of Health Clinical Center in Bethesda, Maryland. It involves the enrolment of at least 53 healthy adults between the ages of 18 and 70 with the first five participants ranging between the ages of 18 and 40. The first five participants will receive a single 20 microgram dose of the vaccine and the remaining 48 participants will receive two 60 microgram vaccinations over a 16 week period. Researchers will study the participants' immune responses to the experimental vaccine and how that response varies with age.
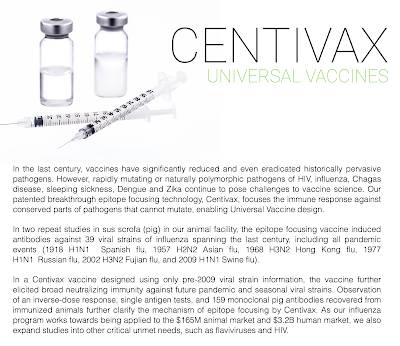
This is not the only attempt to create a universal influenza vaccine. Distributed Bio, a San Francisco-based company is also researching a universal vaccine in their Centivax program as shown here:
Distributed Bio, which receives funding from the Bill & Melinda Gates Foundation (among others), is targeting the regions of the influenza virus that cannot mutate and are using pigs as their medium. Centivax Flu, Distributed Bio's universal influenza vaccine, is slated for its first in-human studies in 2021.
Given the nature of the 2020 influenza panic that is taking place, the development of a universal vaccine is critical before the world experiences another deadly pandemic like the Spanish H1N1 influenza pandemic.
Click HERE to read more from this author.
You can publish this article on your website as long as you provide a link back to this page.


Be the first to comment