
Governments keep assuring us that it is safe to get vaccinated with the "fully tested COVID-19 vaccines" that were developed in record time to beat back the pandemic. Without thinking too deeply about it, one might believe their anti-unvaxxed rhetoric if it weren't for the fact that information which can be gleaned from various government and scientific websites showing this to be at least questionable and, at the worst, a complete fabrication. In this posting, we'll look at several different sources showing the timelines for the development of vaccines throughout history and compare it to the current situation for the vaccines being administered during the pandemic.
Let's start with this video from Canada's federal government about the COVID-19 vaccines and why you should trust the government's approval of the products:
Here's Canada's taxpayer funded CBC (aka the Coronavirus Brainwashing Corporation) weighing in on the subject of mixing and matching COVID-19 vaccine doses:
Note the comment by Canada's Chief Public Health Officer, Theresa Tam, that "…recommendations undergo evolution over time and this is the next stage of the increased understanding of our vaccines."
Let's look at several different sources which will clearly show us just how novel the process has been for the rollout of the COVID-19 vaccines.
1.) Here's what the Canadian government has to say about the development of vaccines (in general) as found on the nation's Library of Parliament HillNotes website which was posted in June 2020 and revised in November 2020:
Notice that according to the Canadian government it can take between 9 and 15 years to complete all three phases of a clinical trial.
Here is the key quote:
"Once an appropriate immune response is detected, the candidate vaccine moves into three phases of human clinical trials to test its safety and efficacy. Each clinical trial phase for a vaccine can still take several years or more to complete."
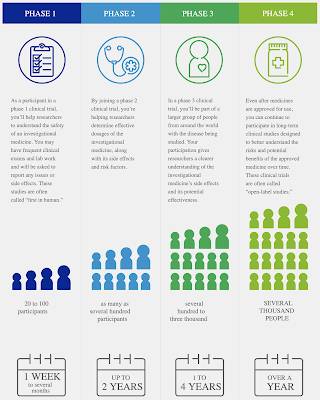
2.) Here is a graphic from Pfizer showing how pharmaceutical trials work:
What is important to note in this graphic is that Phase 3 trials involve between several hundred to three thousand people and Phase 4 trials involve several thousand people. Such is not the case in the current "trials" of the COVID-19 vaccines where hundreds of millions of test subjects have been given up to three doses of the newly minted vaccines.
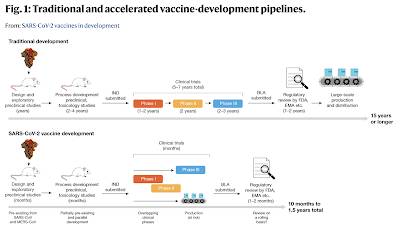
3.) A paper that appeared in Nature back in September 2020 entitled "Traditional and accelerated vaccine-development pipelines" by Florian Krammer provides an interesting comparison of normal vaccine development timelines and the timeline for development of the COVID_19 vaccines as shown here:
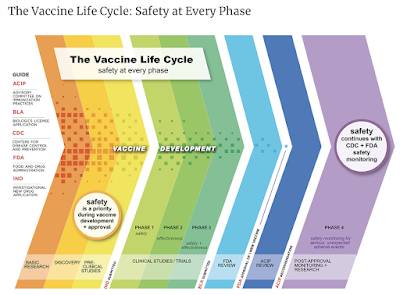
4.) Here is a graphic from the Centers for Disease Control and Prevention showing the "Vaccine Life Cycle" which doesn't show the actual time periods required for each phase of the trial but does show Phase 4 which takes place after FDA approval:
The CDC does state this about the development of vaccines:
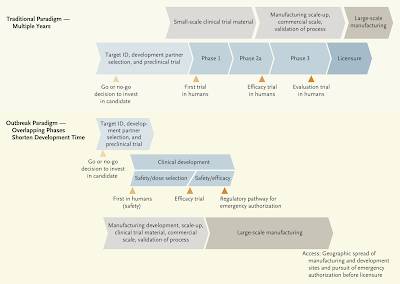
5.) A paper entitled "Developing COVID-19 Vaccines at Pandemic Speed by Nicole Lurie et al which appeared in the New England Journal of Medicine in March 2020 actually provides us with an interesting comparison of the traditional vaccine development paradigm and the paradigm used during a pandemic which clearly shows where changes to the normal process occur:
Here is a quote from the paper:
"Vaccine development is a lengthy, expensive process. Attrition is high, and it typically takes multiple candidates and many years to produce a licensed vaccine. Because of the cost and high failure rates, developers typically follow a linear sequence of steps, with multiple pauses for data analysis or manufacturing-process checks. Developing a vaccine quickly requires a new pandemic paradigm (see diagram), with a fast start and many steps executed in parallel before confirming a successful outcome of another step, hence resulting in elevated financial risk. For example, for platforms with experience in humans, phase 1 clinical trials may be able to proceed in parallel with testing in animal models."
One thing that the authors of the paper do not mention is that the current crop of COVID-19 vaccines still could prove to be failures at stopping the transmission of the virus as well as reducing hospitalizations and the occurrence of serious cases. While some researchers are stating that the vaccines are reducing serious cases, the data is still incomplete and with the rise of the Delta variant, all bets are off.
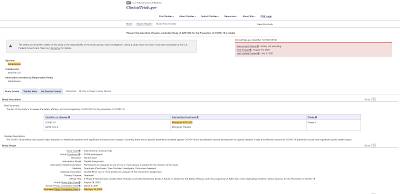
Now, let's look at the facts as they currently stand. According to the manufacturers of the current COVID-19 vaccines, the trials are not even close to being completed:
1.) PfizerBioNTech – Phase 3 to be completed on May 2, 2023 (estimated)
2.) Moderna – Phase 3 to be completed on October 27, 2022 (estimated)
3.) Johnson & Johnson/Janssen – Phase 3 to be completed on January 2, 2023 (estimated)
4.) AstraZeneca – Phase 3 to be completed on February 14, 2023 (estimated)
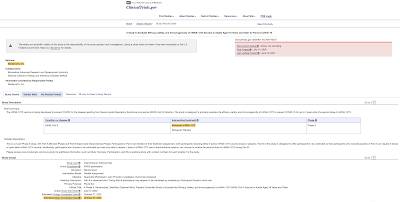
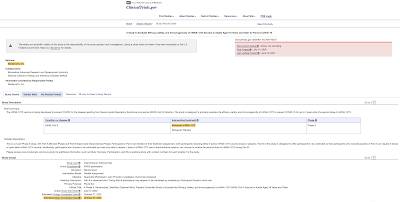
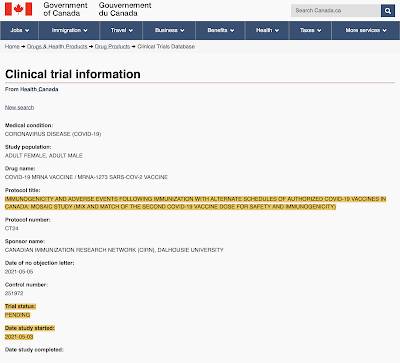
Now, let's look at Canada's "mix and match" vaccine strategy. Actually, there are plans to study this issue as shown here:
…however, the trial status is "pending" and the study only started on May 3, 2021 so, despite what Theresa Tam says, Canadian researchers still have no idea of whether this mixing of vaccines could result in adverse events or whether it will provide immunity to the COVID-19 virus.
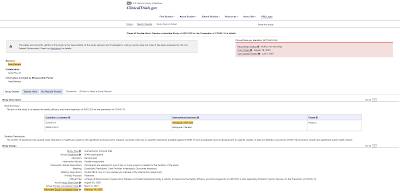
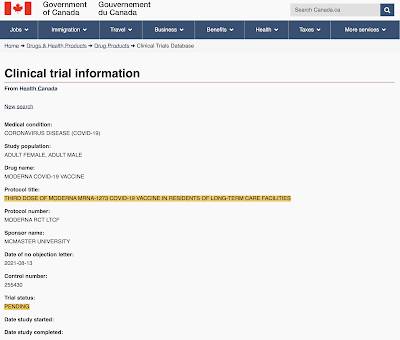
As far as administering a third dose of a COVID-19 vaccine (booster dose), here is the plan to study that issue using residents of long-term care facilities, noting that the study has not even commenced:
Given the fact that proper vaccine testing can take up to a decade and a half to complete through to the end of Phase 3 or Phase 4 trials as I have outlined in this posting and that we still have between 13 and 20 months to complete Phase 3 trials for the Pfizer, Moderna, Johnson & Johnson and AstraZeneca vaccines, how can governments around the world allow Phase 3 trials for the COVID-19 vaccines to be taking place using hundreds of millions of their citizens rather than the several hundred to several thousand participants as would be typical for a Phase 3/Phase 4 trial?
You can publish this article on your website as long as you provide a link back to this page.



Be the first to comment