 Federal Health Minister, Leona Aglukkaq, has initiated an investigation into why effected women were not timely informed, for at least five days, after the officials at Health Canada realized that their birth control contained twice as many placebo pills as advertised. The minister mentioned in an official statement, that “I am concerned that Canadians may not have received important information in a timely manner. As a result, I have instructed Health Canada to look into the issue and assess whether processes were followed and that they are sufficient.”
Federal Health Minister, Leona Aglukkaq, has initiated an investigation into why effected women were not timely informed, for at least five days, after the officials at Health Canada realized that their birth control contained twice as many placebo pills as advertised. The minister mentioned in an official statement, that “I am concerned that Canadians may not have received important information in a timely manner. As a result, I have instructed Health Canada to look into the issue and assess whether processes were followed and that they are sufficient.”
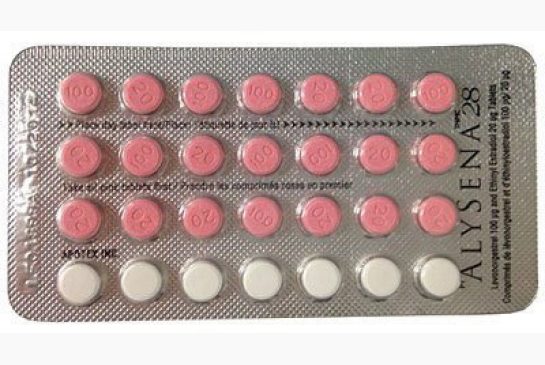
Few thousands women all over Canada were prescribed a defective batch of Alysena 28 contraceptive pills, which constituted of two weeks of sugar pills instead of one. The lot was identified as LF01899A and distributor Apotex issued a voluntary recall to wholesalers and retailers on April 3. Reports show that affected packages were recalled from stores until April 5, however, the effected patients were not contacted up till April 8, i.e. when Health Canada upgraded the recall to Type I.
Health Canada descries that this type of recall is reserved for “a situation in which there is a reasonable probability that the use of, or exposure to, a product will cause serious adverse health consequences or death.” A report elucidated that “(Pregnancy) is considered a Type 1 risk in certain groups of women, such as those who have been advised against becoming pregnant for medical reasons, or in the case of a (sic) unplanned pregnancy when the woman is using certain drug(s) which could be harmful to a developing fetus.”

Be the first to comment