After doing its own studies, Philips is less concerned about the risks of sleep apnea devices.
Previously, the chance of foam particles escaping from Philips’ sleep apnea devices was much higher. Thousands of systems have been tested by external laboratories, and the firm claims this.
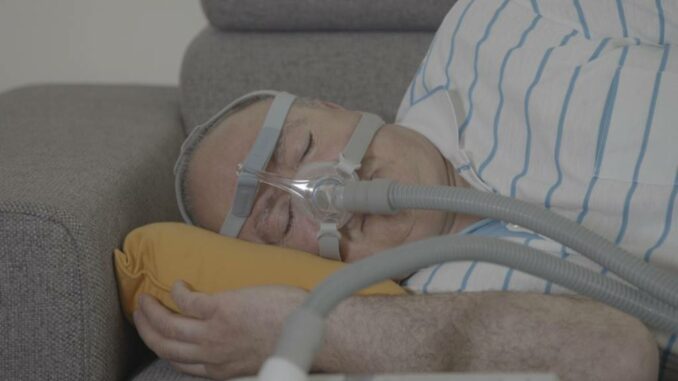
Last year, the business issued a recall. There was a chance that one of the device’s 5.5 million units may leak foam, putting users at danger of inhalation. Modifications are being made to each of those gadgets.
After doing study, Philips has concluded that the danger of foam dislodging is low. A total of 1,360 devices were evaluated across Europe. On any gadget, no foam had come off. More than 60,000 systems in the United States were inspected, and hundreds of systems were found to be defective.
It was determined by both the corporation and independent experts just how hazardous the ejected particles really are. An ageing experiment on foam particles failed. Patients are being studied to discover whether there are any long-term effects.
Please accept our apologies for the extended period of time patients have been forced to wait for this information.
Philips Medical Director Dr. Jan Kimpen
Jan Kimpen, medical director of Philips, stated, “It is really essential that we can finally convey to our patients that we can show a promising result.” Our sincere apologies for keeping patients in suspense for so long.
Kimpen expresses concern about the possibility of foam particles escaping “So far, we haven’t been able to show that it’s bad for the people using it. I’m crossing my fingers that hearing this will at least somewhat comfort the sufferers. Not entirely, as I am unable to provide that level of assurance, but it is an essential first step.”
Many authorities, including the US Food and Drug Administration (FDA), will be looking at the findings of these research in the coming months. Investors will not be relieved by Philips’ study findings. At the start of trading, the value of Philips’ shares dropped by about 4%.
Lawsuits
The outcomes are crucial for Philips because of the numerous lawsuits the company is facing from people all around the world. Some claim that the corporation acted too late and that employees were ill as a result of the company’s use of sleep apnea equipment.
More than 500 patients are represented by Mark de Hek, an attorney. “Many threads remain untied. Although Philips implied that investigations were nearing completion, there is still more work to be done. Patients who are on the fence about utilizing the gadget will be disappointed by this development.” He wants to have his own specialists review the outcomes of Philips’ research, so that he may make his own conclusions.
When it comes to the recall, the Health and Youth Care Inspectorate has already identified “substantially and fundamentally” inadequate procedures.
Philips plans to do further research in the near future to determine the long-term effects of airborne foam particles. Particles are also being examined in newer technologies to determine if they may get loose.

Be the first to comment