This article was last updated on April 16, 2022
Canada: ![]() Oye! Times readers Get FREE $30 to spend on Amazon, Walmart…
Oye! Times readers Get FREE $30 to spend on Amazon, Walmart…
USA: ![]() Oye! Times readers Get FREE $30 to spend on Amazon, Walmart…
Oye! Times readers Get FREE $30 to spend on Amazon, Walmart…
The United States Food and Drug Administration (FDA) has recently approved a third shot or booster of Pfizer's COVID-19 vaccine, Comirnaty, for people at least 65 years old and those that are at risk for severe disease at a recent meeting of the FDA's Vaccines and Related Biological Products Advisory COmmmitee (VRBPAC). At the same meeting, the members of VRBPAC voted against an additional dose of COomirntay for the wider population. With that in mind, let's look at the details of the document supplied to the members of VRBPAC by Pfizer to get a sense of how much testing was actually done on these of a third shot and who could be at risk from the repercussions of this decision.
Let's start with some background information by looking at Pfizer's viewpoint on the waning effectiveness of Comirnaty both over time and by SARS-CoV-2 variant in its submission to the FDA:
Please note that Pfizer admits that the "…data supports the need for a booster (third) dose of Comirntay approximately 6 months after the second dose….for individuals 16 years of age and older". Pfizer also states the following:
"Among vaccinated healthcare workers, decreased neutralizing antibody titers have been associated with vaccinees’ breakthrough infections, along with increased viral load…emerging data suggest that vaccine protection may wane approximately 6 to 8 months following the second dose, and evidence is building to suggest that administration of booster doses of COVID-19 mRNA vaccines is potentially an urgent emerging public health issue."
Thank goodness that Pfizer's profits from Comirnaty have already been "deposited into the bank".
Here is another quote showing how poorly the Comirnaty vaccine performs from an observational study conducted by the State of Israel Ministry of Health with my bold:
"The State of Israel Ministry of Health (Israel MoH) conducted an observational study to assess the effectiveness of BNT162b2 against various SARS-CoV-2 outcomes from 20 June 2021 through 17 July 2021….In this evaluation, among individuals ≥16 years of age, BNT162b2 effectiveness against SARS‐CoV-2 infection was only 39.0% (95% CI: 9.0%, 59.0%) and against symptomatic COVID-19 was 40.5% (95% CI: 8.7%, 61.2%) between 20 June 2021 and 17 July 2021. This was considerably lower than published effectiveness estimates from an earlier time period. Specifically, between 24 January 2021 to 03 April 2021 VE against these same endpoints was greater than or equal to 95 percent."
To address the requirements for a trial of a booster, Pfizer implemented a substudy of the original study supplied to the FDA for the Emergency Use Authorization. This study consisted of the following:
1.) Phase 1 – 23 doubly vaccinated participants between 24 and 75 years of age with 11 of these being between the ages of 18 and 55 and 12 being between the ages of 65 and 75 years. All participants were healthy and were not a high risk for SARS-CoV-2 infection or had antibodies from a previous infection. The Phase 1 cut-off date was May 13, 2021 and no adverse events were recorded after that date.
Instead of being run consecutively as would normally be the case, Pfizer ran Phases 2 and 3 together, a highly unusual practice.
2.) Phase 2/3 – 306 participants aged 19 to 55 years of age (originally 312 participants with four participants withdrawing after receiving their booster with 2 being lost to followup) and 2 who withdrew from the study. The range of duration between the second and third doses was 4.8 to 8.0 months and the median was 6.8 months. This portion of the study was conducted in the United States, Argentina, Brazil Germany, South Africa and Turkey. The Phase 2/3 cut-off date was June 17, 2021 and no adverse events were recorded after that date.
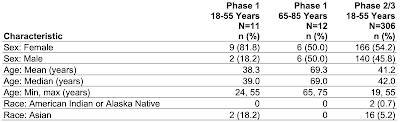
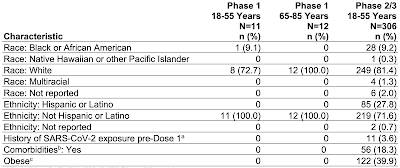
Here is a table showing the demographics of the two trials as screen captured from this document:
In accordance with FDA guidance, the safety and effectiveness of the booster dose given in Phase 2/3 was extrapolated to individuals 16 and 17 years of age and individuals older than 55 years of age as shown here:
Okay, let's let this sink in. The Pfizer booster Phase 1 trial included only 12 older participants between the ages of 65 and 75 with a median age of 69.0 years and a mean (average) age of 69.3 years None of the participants in this age range had comorbidities and none were obese. No Phase 2/3 trial was undertaken for any participant over the age of 55 years; in the case of this trial, the safety and effectiveness of the vaccine was extrapolated from the Phase 2/3 trial results for the younger age group (19 years to 55 years). Basically, the FDA made the decision to allow Pfizer to use a booster shot for Americans aged 65 and older based on a test which included only 12 people with a maximum age of 75 years, no comorbidities and not obese, which will certainly prove to be a challenge in the "real world" given that most Americans over the age of 65 have at least one significant health issue. In addition, the length of the trial was very short at only one month and the effectiveness of the vaccine at improving immunity was also measured only 1 month after completion of the vaccination so we have no idea of what additional protection that a booster will offer as time passes.
As well, Pfizer has made it impossible to follow the medium- and long-term effects of the booster vaccine since they unblinded all of the Phase 2/3 participants in both the vaccine and placebo groups by offering them a booster containing Comirnaty:
I have nothing to add except that I would ask you to ruminate on how the FDA could approve a booster shot for elderly Americans based on a flawed test that included a stacked deck of only 12 individuals between the ages of 65 and 76 years. Do you think that the mainstream media reported this? Not on your life.
This whole thing is turning out to be one giant Big Pharma clusterf@ck.
You can publish this article on your website as long as you provide a link back to this page.





Be the first to comment