Moderna has released the full preliminary results of its clinical trials for its ground-breaking mRNA-1273 vaccine on the New England Journal of Medicine website dated July 14, 2020 and there appears to be some adverse reactions, particularly in the highest doses as you will see in this posting.
The trial which is being sponsored by the National Institute of Allergy and Infectious Diseases (NIAID aka Fauci's Institute) took place with the vaccine being administered in two doses spaced 28 days apart. The three groups received 25-microgram, 100microgram doses and 250-microgram doses. Each group consisted of 15 individuals with the median age being between 31 years and 36.7 years and an age range of between 18 and 55 years. The majority of the participants were white (89 percent) with 13 percent being Hispanic, 4 percent being Black and 2 percent being Asian and American Indian or Alaska Native. Adverse reactions were ranked as mild, moderate or severe.
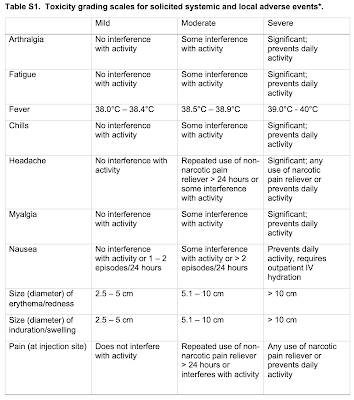
Here is a table showing the adverse reactions and how their severity is measured:
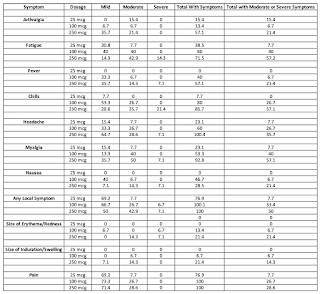
Let's look at the results once the second vaccination took place (in percent with some rounding errors). Arthralgia is joint pain, myalgia is muscle pain and erythema is redness of the skin:
As you can see, in general, the adverse reactions are the worst in the highest dose group (250 microgram) and lowest in the lowest dose group (25 microgram). That said, excluding "any local system", of the ten symptoms, there were 10 times where the administration of the mRNA vaccine resulted in more than 25 percent of the participants experiencing either moderate or severe symptoms and, in two cases, more than half of the participants experienced moderate or severe symptoms, both in the highest dosage group.
Let's close with this quote from the authors of the paper:
"Solicited adverse events that occurred in more than half the participants included fatigue, chills, headache, myalgia, and pain at the injection site. Systemic adverse events were more common after the second vaccination, particularly with the highest dose, and three participants (21%) in the 250-μg dose group reported one or more severe adverse events."
Nonetheless, the authors state that:
"These safety and immunogenicity findings support advancement of the mRNA-1273 vaccine to later-stage clinical trials."
Currently, a Phase 2 trial is taking place with 600 health adults evaluating doses of 50 micrograms and 100 micrograms. A large phase 3 efficacy trial which is expected to evaluate a 100 microgram dose will take place during the summer of 2020.
Click HERE to read more from this author.
You can publish this article on your website as long as you provide a link back to this page.

Be the first to comment