Just in case you thought that Pfizer's mRNA vaccine was the only one to see waning protection, Moderna has now released the results of a new analysis showing how its iteration of the COVID-19 vaccine stands up over time.
Here is the press release with my highlights:
Here are the pertinent highlights with my bolds:
Analysis of Open-Label Part of Phase 3 COVE Study (July-August 2021)
Today, Moderna is sharing a new analysis of the incidence of breakthrough COVID-19 cases among vaccinated participants in the open-label portion of the Phase 3 COVE study between July 1, 2021 and August 27, 2021. The goal of the analysis is to quantify the impact of waning immunity in the face of the Delta surge in the United States. The analysis compared participants initially randomized to mRNA-1273 (vaccinated from July-October 2020; n=14,746; median follow-up of 13 months since first dose) against participants initially randomized to placebo who were crossed over and vaccinated following Emergency Use Authorization (vaccinated from December 2020-March 2021; n=11,431; median follow-up of 8 months since first dose).
In the analysis, 88 breakthrough cases of COVID-19 occurred in the more recently vaccinated group (49.0 cases per 1000 person-years) compared to 162 cases in the group vaccinated last year (77.1 cases per 1000 person-years). The reduction in incidence rates for participants vaccinated more recently compared to participants vaccinated last year was 36% (95% CI: 17-52%). A Cox proportional hazards model showed similar results after adjusting for age and risk factors for severe COVID-19. Fortunately, only 19 severe cases were observed. While not significant, there was a numerical trend towards a lower rate of severe cases in the group vaccinated more recently (3.3 per 1000 person-years) compared to the group vaccinated last year (6.2 per 1000 person-years).
And, here's the "money shot" (literally):
"The increased risk of breakthrough in this analysis quantifies the impact of waning immunity in the COVE study between the median follow-up time of 8 months and 13 months since first dose. The Company believes this adds to evidence of potential benefit of a booster dose of mRNA-1273. A manuscript has been submitted as a preprint to medRxiv and will be submitted for peer-reviewed publication."
This begs the question; how will governments that are insisting upon the issuance of vaccine passports going to handle this? Are the number of vaccines required to receive a passport going to keep increasing as research shows that the "protection" offered by the mRNA vaccines wanes over time?
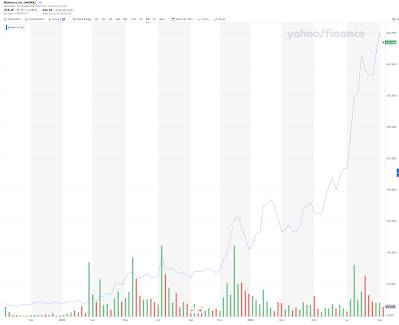
Of course, Moderna has to "keep up with the Joneses" aka Pfizer in advocating for booster doses. After all, what a great way to add billions of dollars of profit to the company's bottom line not to mention billions in wealth to the company's insiders! That way, the mRNA vaccine money printing machine can keep churning out profits for the company's corner office dwellers who have seen Moderna's stock do this over the past five years:
Moderna shareholders and insiders that invested during the company's lean years (stock was sub $20) have now seen a 2170 percent return on their investment. Lucky Bill Gates:
Just for fun and in closing, I read through the "Important Safety Information" on Moderna's press release and found these gems, many of which fly in the face of the advice being touted by public health officials and politicians who are pushing a 100 percent vaccinated mantra:
1.) Immunocompromised persons, including individuals receiving immunosuppressive therapy, may have a diminished response to the Moderna COVID-19 Vaccine.
2.) The Moderna COVID-19 Vaccine may not protect all vaccine recipients.
3.) Available data on Moderna COVID-19 Vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy. Data are not available to assess the effects of Moderna COVID-19 Vaccine on the breastfed infant or on milk production/excretion.
4.) There are no data available on the interchangeability of the Moderna COVID-19 Vaccine with other COVID-19 vaccines to complete the vaccination series. Individuals who have received one dose of Moderna COVID-19 Vaccine should receive a second dose of Moderna COVID-19 Vaccine to complete the vaccination series.
5.) Additional adverse reactions, some of which may be serious, may become apparent with more widespread use of the Moderna COVID-19 Vaccine.
You can publish this article on your website as long as you provide a link back to this page.


Be the first to comment